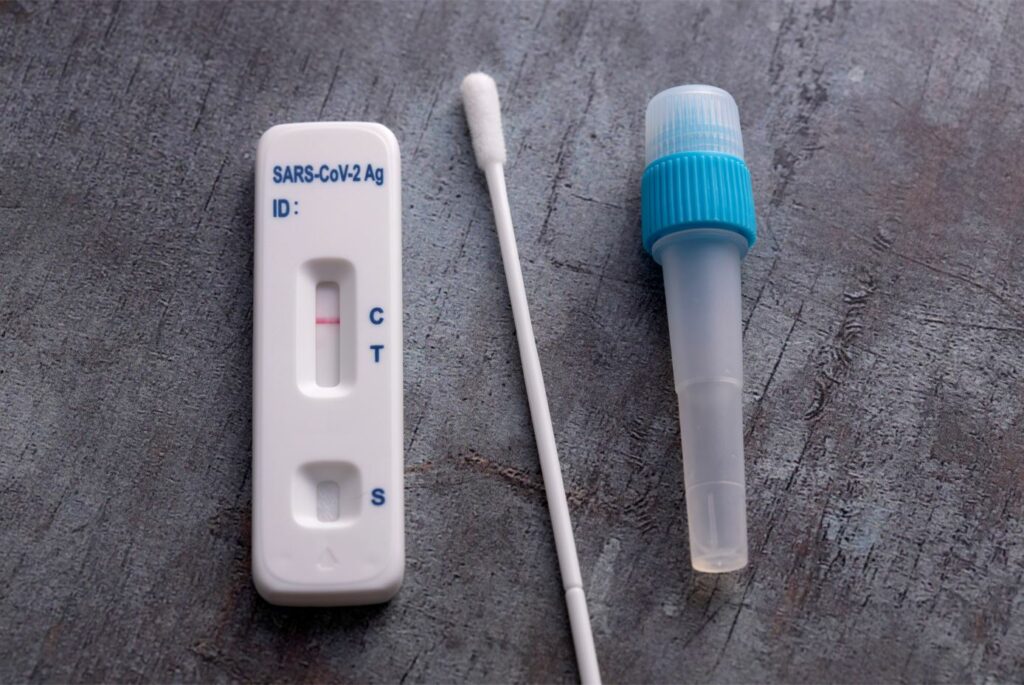
What is a Lateral Flow Rapid Antigen Test?
A SARS-COV-2 lateral flow antigen rapid test is used to quickly process samples on-site without the need for laboratory equipment, generating results in minutes. This kind of rapid diagnostic test is suitable for point-of-care and self-testing (dependant on brand), that directly detects the presence or absence of an antigen. It’s used for the detection of SARS-CoV-2, the virus that causes COVID-19.
Where are Flowflex tests made?
Flowflex is manufactured by Acon Biotech, a global diagnostics company. They are based in the US with manufacturing facilities in the US, China and Mexico.
Where are Healgen tests made?
Healgen is manufactured by Orient Gene, a global diagnostics company. They are based in China, where their production facility is also.
Are Flowflex and Healgen tests approved for use in the UK and Europe?
All medical devises have to follow a standard process before they can be sold in the UK & EU. Both Flowflex and Healgen tests have been through that process and have a CE mark, meaning they can be sold and used in the UK & EU for their intended use.
Flowflex is CE certified as a self-test (home use), and for professional use. Professional use means they can be administered by a healthcare professional, or suitably trained person.
Healgen is CE certified for professional use only, although CE-certified stock for self-tests is imminent.

Are they approved & validated by the Department of Health and Social Care (DHSC), and Public Health England (PHE)?
Yes, both brands are listed and meet the criteria. Since its establishment in August 2020, the joint PHE Porton Down and University of Oxford SARS-CoV-2 lateral flow antigen test validation cell has evaluated over 140 lateral flow devices that have been referred by the Department of Health and Social Care (DHSC).
Approximately 30% of the tests that were referred for validation met the standards for phase 2 validation, which are set out in the protocol. PHE Porton Down subsequently performed phase 3 testing to assess whether the lateral flow devices that passed phase 2 displayed performance characteristics desirable for mass population, community-based testing. The desirable performance characteristics are:
- Very high specificity
- Very high sensitivity against viral loads associated with infectiousness.
Those lateral flow devices that display the desirable performance characteristics are listed within the link below…

What does Sensitivity, Specificity and Accuracy mean in terms of lateral flow tests?
Sensitivity is the proportion of people with the virus who have a positive test.
Specificity is a measure of how good the test is at detecting true negative cases.
Accuracy is a measure of how effective a test is.
Below are the relating percentages for Flowflex and Healgen tests.
Flowflex:
Sensitivity: 97.1% Specificity: 99.6%
Healgen:
Sensitivity: 97.3% Specificity: 99.2%
When should the tests be used?
The Flowflex and Healgen tests pick up moderate to high viral loads. Patients will have moderate to high viral loads about 3 days before symptoms start until between 7 and 10 days after symptoms start. In this window lateral flow tests, such as Flowflex and Healgen are very effective. A positive test should be repeated using a RT-PCR test for confirmation and entry into the national test and trace system. A negative test simply reflects a point in time – you do not have active COVID-19 today.
As of Oct 4th at 4am PCR tests for travel to England are being abolished, and during October they will be replaced with a lateral flow test to be taken within 2 days of entering the country. As lateral flow tests are more cost-effective than PCR this will reduce the costs for testing that travellers have been subject to previoulsy. As Flowflex is CE certied as a self-test it is therefore the ideal product to use for Day 2 testing.
How often should tests be repeated?
This is difficult to advise as advice differs. Bear in mind that a negative result reflects a moment in time. Some bodies have advised testing twice a week, or perhaps every Monday in a standard five day week. Other guidance has suggested testing every day.
What are the Packaging differences between Flowflex and Healgen?
Flowflex comes in multi-packs of 25 tests, multi-packs of 5 tests, and single test packs.
Healgen comes in multi-packs of 20 tests, and singke pack tests.
Worth noting that with Flowflex the extraction tubes come pre-filled with buffer solution, unlike Healgen (in packs of 20), whereby it’s necessary to manually fill the extraction tubes from a master buffer solution bottle.
To find out more visit the product page HERE, or for enquiries and orders please submit the contact form below, or call the team on 0114 2213344.